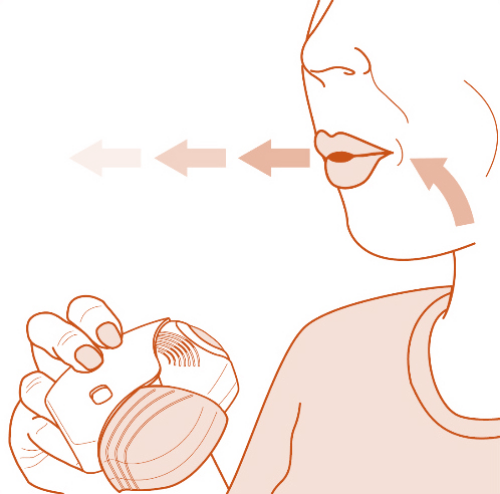
Once-daily ANORO is a prescription medicine used long term to treat chronic obstructive pulmonary disease (COPD), including chronic bronchitis, emphysema, or both, for better breathing and to reduce the number of flare-ups. ANORO is not for asthma. ANORO is not used to relieve sudden symptoms of COPD and won't replace a rescue inhaler.
Once-daily ANORO is a prescription medicine used long term to treat chronic obstructive pulmonary disease (COPD), including chronic bronchitis, emphysema, or both, for better breathing and to reduce the number of flare-ups. ANORO is not for asthma. ANORO is not used to relieve sudden symptoms of COPD and won't replace a rescue inhaler.
Once-daily ANORO is a prescription medicine used long term to treat chronic obstructive pulmonary disease (COPD), including chronic bronchitis, emphysema, or both, for better breathing and to reduce the number of flare-ups. ANORO is not for asthma. ANORO is not used to relieve sudden symptoms of COPD and won't replace a rescue inhaler.
- Do not use ANORO to treat sudden symptoms of COPD. Always have a rescue inhaler with you to treat sudden symptoms.
- Do not use ANORO if you have a severe allergy to milk proteins or are allergic to any of the ingredients in ANORO. Ask your healthcare provider if you are not sure.
- Do not use ANORO to treat sudden symptoms of COPD. Always have a rescue inhaler with you to treat sudden symptoms.
- Do not use ANORO if you have a severe allergy to milk proteins or are allergic to any of the ingredients in ANORO. Ask your healthcare provider if you are not sure.
- Do not use ANORO to treat sudden symptoms of COPD. Always have a rescue inhaler with you to treat sudden symptoms.
- Do not use ANORO if you have a severe allergy to milk proteins or are allergic to any of the ingredients in ANORO. Ask your healthcare provider if you are not sure.
- Do not use ANORO if you have asthma.
- Do not use ANORO more often than prescribed.
- Do not take ANORO with other medicines that contain a long-acting beta2-adrenergic agonist (LABA) or an anticholinergic for any reason. Tell your healthcare provider about all your medical conditions and about all the medicines you take.
- Call your healthcare provider or get medical care right away if your breathing problems get worse, if you need to use your rescue inhaler more often than usual, or if your rescue inhaler does not work as well to relieve your symptoms.
- ANORO can cause serious side effects, including:
- COPD symptoms that get worse over time. If this happens, do not increase your dose of ANORO; instead, call your healthcare provider.
- symptoms of using too much of a LABA medicine, including: chest pain; increased blood pressure; fast or irregular heartbeat; headache; tremor; nervousness.
- sudden breathing problems immediately after inhaling your medicine. If you experience this, stop using ANORO and call your healthcare provider right away.
- serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms: rash; swelling of your face, mouth, and tongue; hives; breathing problems.
- effects on heart: increased blood pressure; chest pain; a fast or irregular heartbeat; awareness of heartbeat.
- effects on nervous system: tremor; nervousness.
- new or worsening eye problems including acute narrow-angle glaucoma that can cause permanent loss of vision if not treated. You should have regular eye exams while using ANORO. Symptoms may include: eye pain or discomfort; nausea or vomiting; blurred vision; seeing halos or bright colors around lights; red eyes. If you have these symptoms, call your healthcare provider right away before taking another dose.
- urinary retention. People who take ANORO may develop new or worse urinary retention. Symptoms may include: difficulty urinating; painful urination; urinating frequently; urination in a weak stream or drips. If you have these symptoms, stop taking ANORO and call your healthcare provider right away before taking another dose.
- changes in laboratory blood values, including high levels of blood sugar and low levels of potassium.
- Common side effects of ANORO include: sore throat; sinus infection; lower respiratory infection; common cold symptoms; constipation; diarrhea; pain in your arms or legs; muscle spasms; neck pain; chest pain.